
Acidic Water
Click the Treatment Menu above for additional Water Issues and Solutions!
OVERVIEW AND TREATMENT OPTIONS:
The potential of Hydrogen (pH) is a measure of the alkaline or acid content in your water. Normal drinking water pH ranges between 6 – 8.5 with neutral pH at 7. There are a number of factors that will lower the pH value of your water, Water with low pH is considered acidic water. This is corrosive and will can cause copper pipes to pit & corrode, and may lead to leaching of toxic metals such as lead from pipes and/or fixtures. It can also leave a bluish green stain on your fixtures, sinks, toilets, and household plumbing. Acid Neutralizers can be used to feed the water supply and effectively raise the pH.
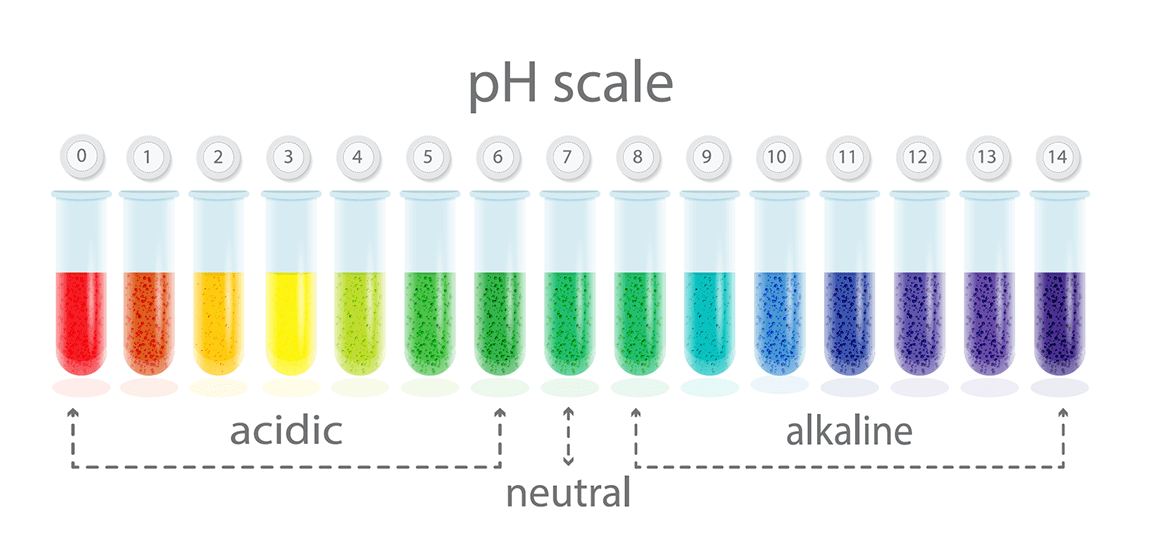
Acidic water is water with a pH lower than neutral (see scale below). This is a measure of the activity of hydrogen ions (H+) in that solution. In essence, it’s a measurement of how acidic or basic a solution is. By looking at the scale below, you see the pH ranges from 0 – 14 with the lower numbers relating to the acidic side of the scale.
Water with a pH that is lower than 7 is considered acidic, and this type of water is more likely to corrode metal pipes and leach metals out of whatever it’s exposed to. Water with a pH that is greater than 7 falls on the alkaline side of the scale. Alkalinity is a measure of the capacity of the water to resist a change in pH that would tend to make the water more acidic. The measurement of alkalinity is needed to determine the corrosiveness of the water.
Remember that the pH scale is logarithmic meaning that an increase or decrease of an integer value changes the concentration by tenfold. So water with a pH of 5.0 is ten more acidic than 6.0, yet it’s 100 times more acidic than 7.0

SOURCES
Acidic water can occur naturally, or it may be caused by a high level of dissolved oxygen. Acidic water is typically low in calcium minerals but high in dissolved carbon dioxide. This can cause the pH to become low and acidic. But there are many reasons that can cause the pH in drinking water to be low, including acid rain. Tree roots, rock formations, or soil microbes can all cause a nearby water supply to become acidic as well.
TESTING
The pipes in most water distribution systems would normally show the existence of water containing a low pH. Since acidic water will leach metals from piping distribution networks, it is wise to test for heavy metals such as lead, chromium, & copper. If testing your water indicates that you have a high pH, consider testing for hardness and alkalinity, since these are closely related to the water type as well.
HEALTH CONCERNS
The largest health issue with acidic water would mostly be related to copper pipes. Acidic water can dissolve some of the copper from the pipes, then this can be consumed in your drinking water. Small amounts of copper are actually good for our diets, but long-term exposure to high amounts of copper could cause serious health problems such as kidney or liver damage. Short-term exposure over the MCL can cause stomach problems, including nausea and vomiting.
TREATMENT
When corrections to pH need to be made, an acid neutralizer filter is typically used to adjust the lower levels. Calcite, also known as a calcium carbonate, is the most common media that is used for these applications. Corosex, magnesium oxide, is often added for extremely low and aggressive pH levels. CLICK HERE to check the BWS line of neutralizers.

