
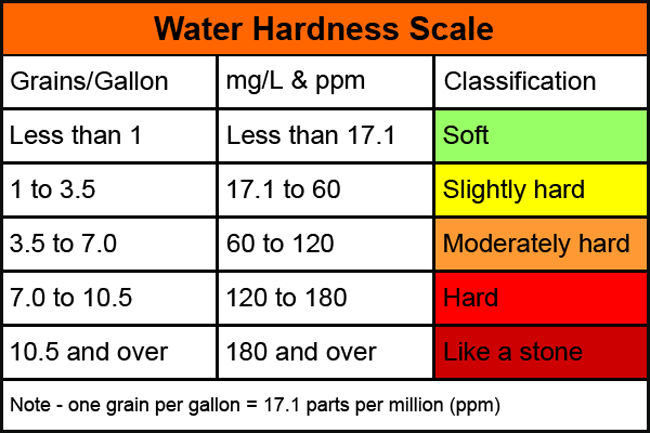
Hardness
 OVERVIEW AND TREATMENT OPTIONS:
OVERVIEW AND TREATMENT OPTIONS:
Water hardness is the amount of dissolved calcium and magnesium in the water, generally having high content of these dissolved minerals. You will notice hardness by lack of suds while washing your hands or bathing, residue and/or spots left on your dishes or car. The term hardness was originally applied to water supplies that were hard to wash in, referring to the soap wasting properties of hard water. In hard water, soap reacts with the calcium to form what is called “soap scum”. Using water that is hard will require more soap or detergent to get things clean, regardless if its your hands, hair, or laundry. CLICK HERE for a quick guide to why you may need a softener.
EFFECTS
Hard water can cause increased soap consumption and leave deposits of scale in water distribution systems. Excessively hard water can also be corrosive. Corrosion can be associated with health risks leaching metals such as lead and copper from pipes, and reduces lifespan of the water system and household appliances that use water.
SOURCES
The principle source of hardness in water are dissolved polyvalent metallic ions from sedimentary rocks, mainly calcium and magnesium. Seepage and runoff from soils of these minerals mainly come from limestone and chalk. Other minor contribution’s to the total hardness would include aluminum, barium, iron, manganese, strontium, and zinc.
HEALTH CONCERNS
Calcium and magnesium are both essential minerals that are beneficial to human health. Inadequate intake of either could lead to adverse results. Inadequate intakes of calcium have been associated with osteoporosis, colorectal cancer, coronary artery disease, resistance to insulin and obesity. Magnesium helps the body synthesize proteins and nucleic acids. It is also a cofactor for nearly 350 cellular enzymes, most of which directly corelate in energy metabolism. Exposure to hard water has also been suggested to exasperate the effects of eczema. A possible explanation is that increased soap usage in hard water results in metal or soap salt residues on the skin or clothes that aren’t easily rinsed off, leading to contact irritation (Thomas & Sach, 2000).
TREATMENT
Point-of-entry ion exchange systems (WATER SOFTENERS) are most commonly used to remove hardness (calcium & magnesium) at the same time reducing the iron and manganese in the water system. Ionic exchange occurs when each divalent ion in the water is replaced with two sodium ions. Softened water will help reduce scaling in the household pipes, fixtures and water heaters. It will improve laundry efficiency helping equipment to not work so hard and making clothes whiter and cleaner. Its adverse effect would be increasing the concentration of sodium & chlorides.
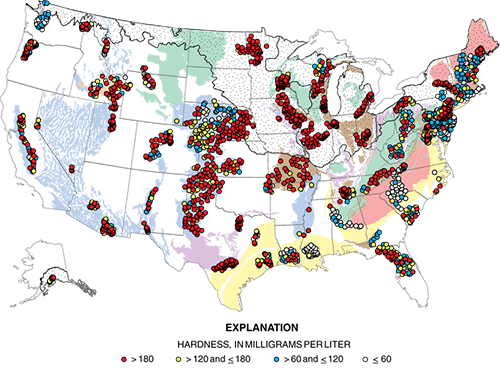
Hardness of groundwater from domestic wells, a USGS study
A study from the National Water-Quality Assessment (NAWQA) Project assessed water-quality conditions for about 2,100 domestic wells across the United States. Water hardness was one water-quality parameter studied; results are shown in the map to the left.

